-
Ingredient SolutionsQuickly narrow your search. Browse products in our sortable catalog. View Catalog
-
ApplicationsQuickly narrow your search. Browse products in our sortable catalog. View Catalog
-
ResourcesRecently Posted
-
PLT People & Planet
-
About
Our international network, passionate team of experts and extensive industry knowledge is what sets us apart.
 Seth FlowermanCEO
Seth FlowermanCEO
AlvioLife®

OVERVIEW
AlvioLife® is a proprietary composition of herbal and fruit extracts designed to support enhanced lung function, improved immune health and an improved general sense of well-being.

UP TO 16%
Improved lung function^
UP TO 7%
Improved aerobic exercise capacity^

UP TO 39%
Reduction in upper respiratory symptoms^
UP TO 18%
Improvement in perceived well-being^
FEATURES & BENEFITS
Clinically studied |
|
Improvements as fast as 3 weeks* |
|
Improves lung function up to 16%* |
|
Improves aerobic exercise capacity by 7%* |
|
Reduces upper respiratory symptoms up to 39%* |
|
Improves subjective well-being by 18%* |
|
Supports immune function |
|
Reduces cold complaints |
|
Low, 200mg/day dose |

CERTIFICATIONS
VEGAN
GLUTEN-FREE
NON-GMO
HALAL
KOSHER
A Synergistic Botanical Complex
AlvioLife is a patented composition of Boswellia serrata resin standardized to 30% 3-O-acetyl-11-keto-ß-boswellic acid (AKBA) and Bengal quince fruit extracts (Aegle marmelos).
Boswellia serrata
Sourced from the forests of India, Boswellia has been used in Ayurvedic medicine for thousands of years.

Aegle marmelos
Known as Bengal quince fruit, it is a species of tree native to the Indian subcontinent and Southeast Asia.
Rhodiola rosea
Rhodiola rosea is a plant native to remote Arctic climates in Asia, Europe and North America.

Exciting Phytochemistry
The root of the plant contains around 140 chemical compounds including phenols, rosavin, rosarin, salidroside and more.
Detail 1
Kale chips cold-pressed put a bird on it mumblecore kogi brooklyn farm-to-table blue bottle yuccie authentic kombucha migas. Literally tilde tacos paleo.

Detail 2
Kale chips cold-pressed put a bird on it mumblecore kogi brooklyn farm-to-table blue bottle yuccie authentic kombucha migas. Literally tilde tacos paleo.
RESEARCH
Overall Respiratory Support. Improved Immune Function.
Better Quality of Life.
AlvioLife has been the subject of two human clinical trials measuring respiratory function.
Results from Second AlvioLife Clinical Study
CLINICAL HIGHLIGHTS
Clinical Study: Easier breathing starting at 14 days
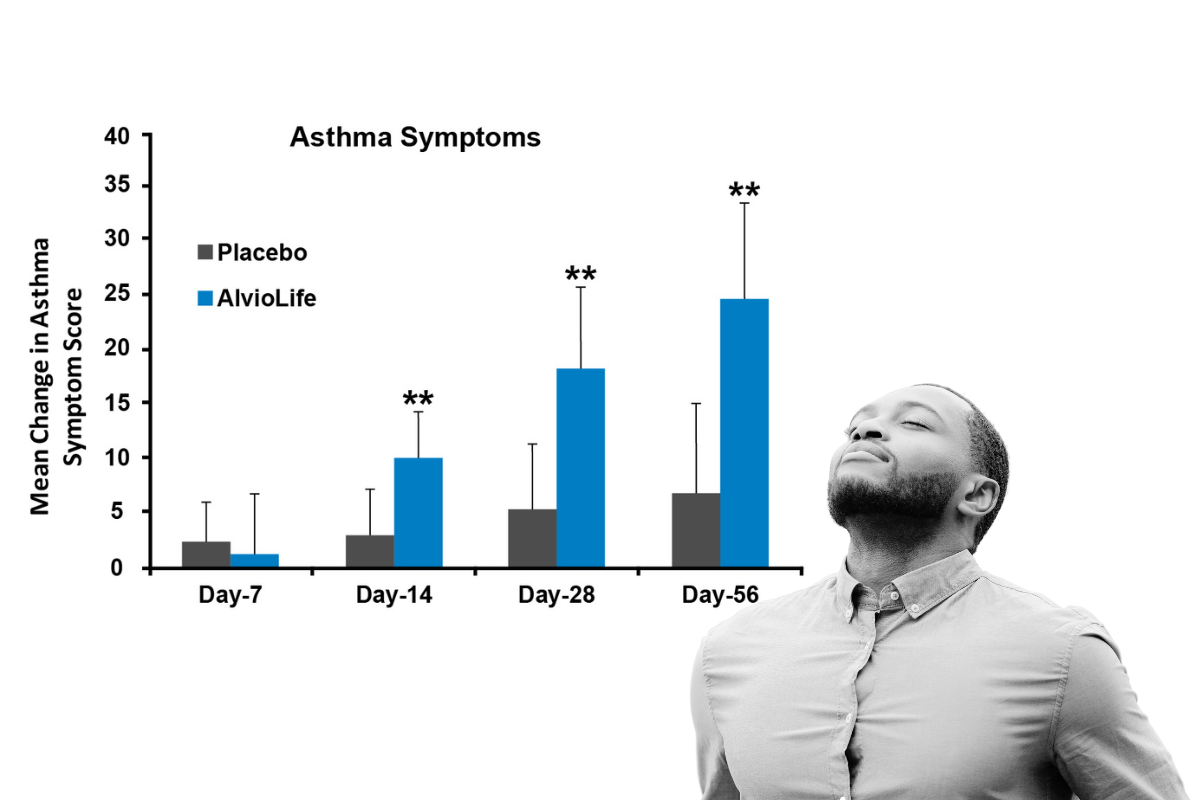
Thirty-six subjects took AlvioLife (200 mg/d) or a matching placebo for 56 days. AlvioLife conferred significant improvements in emotional function (p = .0305) and asthma symptoms scores (p = .0002) were improved even at 14 days, compared with the placebo. 56 days of supplementation of AlvioLife resulted in a significant increase in serum IFN‐γ (p = .0014) and a reduction in IL‐4 (p = .0497), compared with placebo. Yugandhar P, Rao KM, Sengupta K. A novel herbal composition containing extracts of Boswellia serrata gum resin and Aegle marmelos fruit alleviates symptoms of asthma in a placebo controlled double-blind clinical study.
Phytother Res. 2018;32(1):140-150. doi:10.1002/ptr.5963

Clinical Study: AlvioLife improves lung function and lung capacity
.png)
AlvioLife was found to improve lung function (FEV1) by up to 16%; lung capacity (FVC) by up to 30%; aerobic exercise capacity by up to 7% (6-minute Walk test); to reduce upper respiratory tract symptoms (WURSS-21) by up to ~40%; improved perceived immune status (ISQ) in as early as three weeks; improved psychological well-being (PGWBI) by up to 18%; and improved the percentage of CD4+ T Helper Cells. In Publication pending)
.png)
MARKET OPPORTUNITIES
Respiratory Health
AlvioLife offers broad spectrum support for people’s respiratory health including improved lung function and capacity.
Active Lifestyle
Studies with AlvioLife show support of aerobic exercise capacity and exercise tolerance – increasing walking distance and speed.
Immune Health
Studies show that AlvioLife reduces upper respiratory tract symptoms and also reduces the frequency of cold and flu complaints.
Cognitive Health
Clinical studies with AlvioLife show an improved sense of well-being and healthy psychological wellness.
ORIGIN STORY
A Sustainable and Socially Responsible Ingredient
Bengal quince fruit (Aegle marmelos) is a species of tree native to the Indian subcontinent and Southeast Asia. The tree is considered to be sacred by Hindus and is often found near temples and home gardens.

Tree Populations Safety and Health
PLT’s Boswellia serrata supply chain starts with collectors and aggregators who have worked with us for decades. These collectors are trained in sustainable gum tapping to maximize yield while maintaining the safety and health of the Boswellia serrata tree populations.

Botanical Liaisons, LLC
PLT has started a third-party sustainability audit program on Boswellia serrata trees in India by renowned botanical research consultancy Botanical Liaisons, LLC. The third-party audit program is based on a broad range of environmental, cultural and economic parameters.

Strong Practices
At PLT, we understand that proper stewardship of natural resources and social responsibility toward the communities that support our work is both the right thing to do and good business.

SUSTAINABILITY
Understanding the Difference Makes the Difference
During the fourth quarter of 2021, PLT Health Solutions announced the commencement of a third-party sustainability audit program on Boswellia serrata trees, which serve as the raw material source for some of our most important ingredients. The audit of this India resource was conducted by renowned botanical research consultancy Botanical Liaisons, LLC, and was undertaken to help the nutraceutical industry and non-governmental agencies around the world understand what we and our ingredient innovation partner Laila Nutraceuticals have known for many years: proper stewardship of natural resources and social responsibility toward the communities that support our work are both the right thing to do and good business.
The audit program was a step in PLT’s campaign to document the sustainability of the tree resource. PLT’s Chairman, Paul Flowerman, made field trips to the key production areas in 2018 and 2019 to observe and document the variety of tree populations, methods of natural and forced propagation and the status of the tribal groups involved in the care of the trees and resin production.


A third audit program was based on a broad range of environmental, cultural and economic parameters. In March 2022 Botanical Liaisons reported to PLT its preliminary conclusions, which included this statement: “It is clear from the information collected from the stakeholders that [sustainability] is supported. Boswellia serrata has several sustainability advantages that prevent over-harvesting of PLT-sourced Boswellia compared to other sources and species of Boswellia.” The report recommended a series of next steps to further evaluate and promote sustainability.
Subsequently, four important additional developments have added considerable strength to the Boswellia serrata sustainability story:
- Authoritative tree survey data from Madhya Pradesh quantified the robustness and sufficiency of the tree resource to provide present and future resin requirements.
- CITES (Convention on International Trade in Endangered Species of Wild Fauna and Flora) determined during its November 2022 meeting (COP 19 in Panama City) that no Boswellia species would get added to the CITES Appendix lists. The health of Boswellia serrata, the only species of commercial importance for PLT, was documented, including information about the replenishment of the resource.
- AHPA (American Herbal Products Association) convened an expert panel, which has drafted a brochure entitled, “Good Stewardship Harvesting of Boswellia (Boswellia Serrata).” This is a definitive presentation of current best practices.
- An important study modeling the impact of significant climate change by 2050 on Boswellia serrata resources predicted that almost all of Madhya Pradesh, the source of 90+% of commercial resin, will remain conducive for a healthy tree population.
These initiatives represent the type of work we do with our PLT360™ initiative. Introduced in 2015, our PLT360 initiative is a business-wide commitment by PLT Health Solutions to develop ingredients that our customers can be confident and proud to supply to their own customers – knowing that these ingredients are safe, of high quality, efficacious and harvested and manufactured sustainably. PLT360 examines every business decision and business process we undertake to be transparent with our operations, build trust with the health & well-being community and, together with them, support healthier, happier lives for the consumers we serve.
INSIGHTS

PLT Receives License for AlvioLife from Health Canada
In 2020, PLT received a license from the Natural and Non-Prescription Health Products Directorate (NNHPD) of Health Canada to market AlvioLife to support respiratory health in Canada. Published clinical work has shown that it can help improve overall respiratory health, maintain clear airways, help against environmental exposure and soothe respiratory tissues.
In Canada, the approved claims include:
• AlvioLife helps to support lower respiratory tract health
• AlvioLife helps to support lower respiratory tract health that includes the bronchial tubes and the lungs’ health
• Supports lower respiratory system function
• Clinically shown to support healthy lower respiratory system function

“It’s easy to forget how many aspects of our lives are dependent on respiratory health – from overall health to emotional well-being to physical performance.”
Seth Flowerman, President & CEO, PLT Health Solutions
RESOURCES



PLT Health Solutions, Inc. announced that it has received an expanded license from the Natural and Non-Prescription Health Products Directorate (NNHPD) of Health Canada to market its ingredient AlvioLife® to support respiratory health in Canada.

Check Out an Ingredient Whose Time Has Come
Clinically studied AlvioLife is a top ingredient in respiratory health and can be a component in your immune health formulations.
- Expertise
- Ingredient Solutions
- All
- Animal Health & Wellness
- Beauty from Within
- Joint & Bone
- Cardiovascular
- Cognitive Performance
- Energy
- Functional Foods & Beverages
- Healthy Aging & Longevity
- Hydration+
- Immune & Respiratory Health
- Men’s Health
- Muscle Health
- Pain & Mobility
- Plant-Based Nutrition
- Sexual Health
- Sleep
- Sports & Active Nutrition
- Stress & Mood
- Weight Management
- Women’s Health
- Applications
- Resources
- PLT People & Planet
- About
These products are not intended to diagnose, treat, cure or prevent disease. This website is for informational purposes only.



















