-
Ingredient SolutionsQuickly narrow your search. Browse products in our sortable catalog. View Catalog
-
ApplicationsQuickly narrow your search. Browse products in our sortable catalog. View Catalog
-
ResourcesRecently Posted
-
PLT People & Planet
-
About
Our international network, passionate team of experts and extensive industry knowledge is what sets us apart.
 Seth FlowermanCEO
Seth FlowermanCEO
Rhodiolife® Rhodiola rosea

OVERVIEW
Rhodiolife generates sustained energy and promotes wellness including psychological, emotional and physical conditioning.
AWARDS

3 Published Studies
Rhodiolife is backed by science that supports its benefits.
Fingerprint Matters
Only Rhodiolife’s unique “fingerprint” composition consistently provides the spectrum of compounds found in the root of the plant.

CITES Compliant
CITES compliant Rhodiolife is now sourced from both wildcrafted and cultivated raw materials.

3rd Party Verified
Rhodiolife is the world’s first Rhodiola rosea third-party verified for ingredient identity and sustainable sourcing.
Help your customers achieve balance
More than ever, people are tuning to mental and physical well-being as we navigate a ‘new normal’. Today, adaptogens are an increasingly sought-after class of ingredient. Rhodiolife Rhodiola rosea is a true adaptogen with clinical science and a great origin story.
PLT and Nektium's Partnership at Work
PLT works with some of the most innovative, socially-committed companies in the world in bringing our solutions for health & wellness to market. Nektium from Canary Islands is a unique company with a fascinating story to tell your customers.
FEATURES & BENEFITS
Consistent, standardized extracts |
|
Cultivation program in place |
|
Sustainable, socially responsible |
|
Full ID assessment and traceability |
|
Sourced from Altai Mountains |
|
Secure supply |
|
3 published clinical studies |
|
Self-affirmed GRAS |
|
Water-soluble |
|
Non-GMO Project Verified |
|
|
|

CERTIFICATIONS
CLINICAL SCIENCE
KOSHER
HALAL
NON-GMO
GRAS
BSCG CERTIFIED FOR SPORTS
3RD PARTY VERIFIED SUSTAINABLE
A True Adaptogen. Standardized. Sustainable.
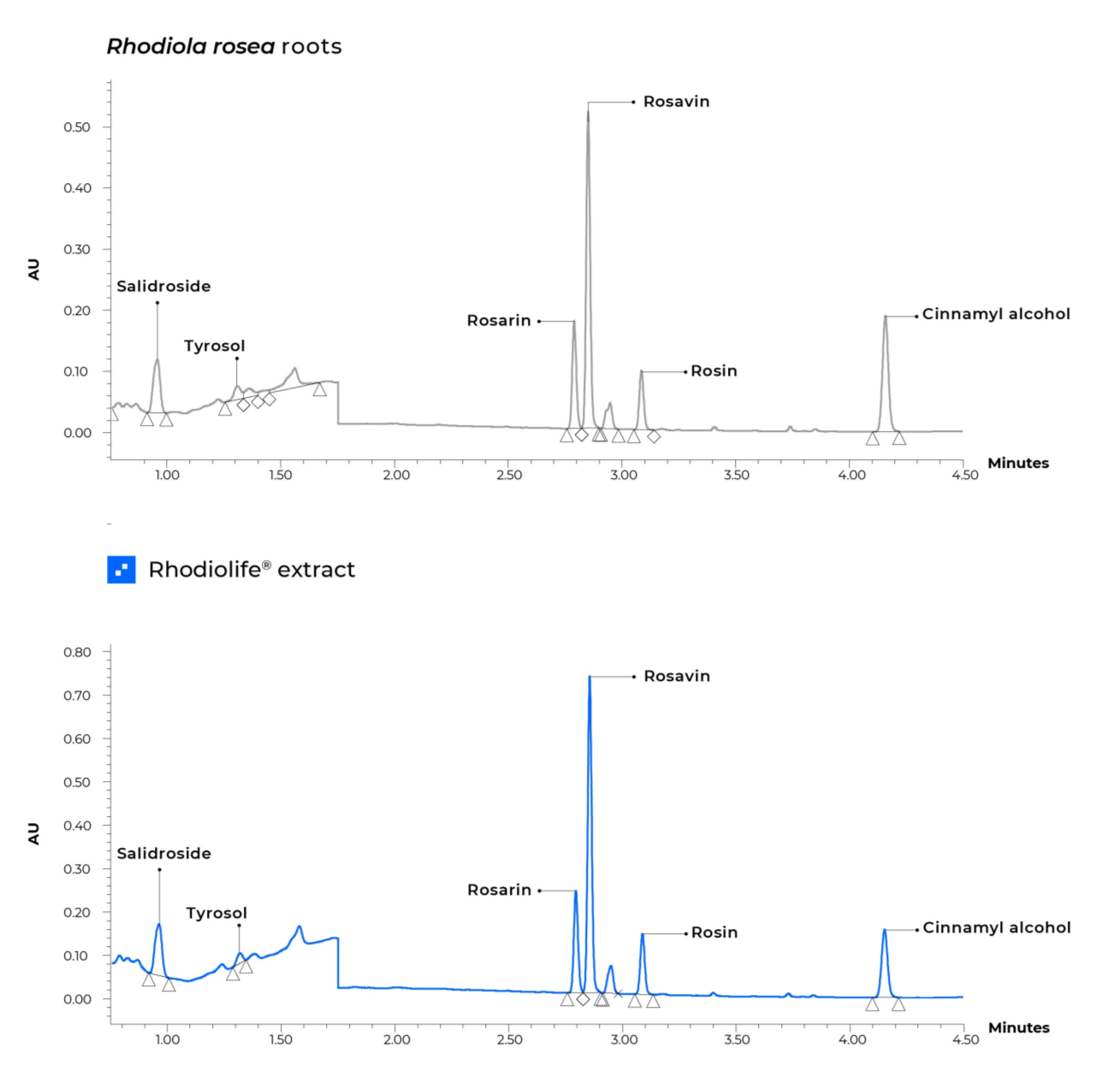
Rhodiolife is a standardized extract of Rhodiola rosea – which is considered a true adaptogen. Only Rhodiolife’s unique “fingerprint” composition consistently provides the spectrum of compounds found in the root of the plant that are responsible for its biological activity .
Rhodiola rosea
Rhodiola rosea is a plant native to remote Arctic climates in Asia, Europe and North America.

Exciting Phytochemistry
The root of the plant contains around 140 chemical compounds including phenols, rosavin, rosarin, salidroside and more.
Rhodiola rosea
Rhodiola rosea is a plant native to remote Arctic climates in Asia, Europe and North America.

Exciting Phytochemistry
The root of the plant contains around 140 chemical compounds including phenols, rosavin, rosarin, salidroside and more.
Detail 1
Kale chips cold-pressed put a bird on it mumblecore kogi brooklyn farm-to-table blue bottle yuccie authentic kombucha migas. Literally tilde tacos paleo.

Detail 2
Kale chips cold-pressed put a bird on it mumblecore kogi brooklyn farm-to-table blue bottle yuccie authentic kombucha migas. Literally tilde tacos paleo.
Complementing the body of published work on Rhodiola rosea, preclinical and clinical trials using Rhodiolife have been conducted and publishe
Rhodiolife increases physical working capacity - pre-clinical
Design
• 24 Sprague-Dawley Rats given R. rosea (Rhodiolife), R. crenulata, or control via oral gavage (50 mg/kg)
• Twice-daily exhaustive swimming sessions for six days
• Outcomes measured
• Time to exhaustion
• Skeletal muscle mitochondrial ATP
Results
• Mean time to exhaustion was virtually the same in rats treated with R. crenulata and controls (34.2-35.5 minutes)
• Mean time to exhaustion in Rhodiolife-treated rats was far greater (45 minutes)
• ATP content in the mitochondria decreased after swimming exercise in all animals. However, the decrease in rats receiving Rhodiolife was less pronounced in comparison with not only the control, but also with the R. crenulata group (p<0.05)
In this animal study, the effects of two different Rhodiola extracts were studied on physical performance and muscle health.
The graph shows that rats supplemented with RhodioLife were able to swim almost 25% longer than control or R. crenulata supplemented rats. This may be due in part to the ability of R. rosea extract to activate the synthesis or resynthesis of ATP in mitochondria and stimulate reparative energy processes during and after intense exercise. As shown in Table 1, rats in the RhodioLife supplemented group had less of a decline in muscle ATP than did rats in either of the other 2 groups.
NOTE: Salidroside content in R. crenulata extract was 2.5 times higher than in R. rosea extract. Hence, the effects of R. rosea extract are due to the presence of rosavins and/or their complex with other compounds.
REF: Abidov M, Crendal F, Grachev S, Seifulla R, Ziegenfuss T. Effect of Extracts from Rhodiola Rosea and Rhodiola Crenulata (Crassulaceae) Roots on ATP Content in Mitochondria of Skeletal Muscles. Bull Expo Biol Med. 2003;136(6):585–7.
Rhodiolife produces brain-wave activity similar to ginkgo
Quantitative EEG Discriminant Analysis provides a unique comparison
Design
• Male adult Fischer rats implanted with qEEG electrodes
• Rhodiolife extract administered orally by gavage (100 mg/kg)
• Outcomes measured
• Analysis of spectral power by qEEG
• In vitro hippocampal slice analysis
Results
• Rhodiola attenuated delta and theta power, likely related to interference with the cholinergic and norepinephrinergic transmission, respectively.
• Using discriminant analysis for comparison with reference pharmaceutical and botanical drugs, Rhodiola projected near the position of Ginkgo extract.
Dimpfel W, Schombert L, Vega-Morales T, Wiebe JC. Neuropharmacological Characterization of Extracts from Rhodiola rosea, Oenothera paradoxa and Paullinia cupana in Comparison to Caffeine. Pharmacol Pharm. 2016;7(7). DOI: 10.4236/pp.2016.77036
Rhodiolife supports muscle during exercise
Anti-inflammatory and muscle-protective effects in humans
RhodioLife has also been studied in untrained humans as well. In this study, subjects consumed RhodioLife before and after exhausting physical exercise on an ergometer.
Design
• N=36 healthy, untrained volunteers aged 21-24 years
• Supplemented 30 d prior and 6 d after exhausting physical exercise with:
• Placebo (340 mg twice/day)
• Rhodiola rosea (Rhodiolife, 340 mg twice/day)
• Control (no treatment)
Results
• Less pronounced rise in CRP in the Rhodiolife group. Levels returned to baseline only in this group.
• Creatine Kinase (CK) was also blunted in the Rhodiolife supplemented subjects
Rhodiolife may reduce occasional anxiety
Pilot clinical trial in adults with anxiety
In this small, pilot study, 10 participants with DSM-IV diagnosis of generalized anxiety disorder were supplemented with RhodioLife for 10 weeks. Several assessments to clinically evaluate symptoms at baseline and following supplementation were used.
Design
• N=10 subjects with anxiety
• Open-label design
• Rhodiola rosea (Rhodiolife) 340 mg/d for 10 weeks
• Assessments:
• Hamilton Anxiety Rating Scale (HAM-A or HARS) and Hamilton Depression Rating Scale (HAM-D or HDRS)
• Four-Dimensional Anxiety and Depression Scale (FDADS)
• Clinical Global Impressions of Severity/Improvement Scale
Results
• Daily Rhodiolife consumption decreased ratings on scales of anxiety (HAM-A) and depression (HAM-D). (p=0.001)
• Self-rated scores on FDADS also significantly decreased (p=0.043
*p<0.05 vs control, placebo
Abidov M et al. Bull Exp Biol Med 2004;7:63-4.
A less pronounced rise in the inflammatory marker C-reactive protein was observed in the Rhodiolife supplemented subjects. CRP levels returned to baseline only in this group.
Creatine Kinase, a marker of muscle breakdown or damage was also blunted in the RhodioLife supplemented subjects
1. Exhausting exercise increased both CRP and CK levels in all subjects, but the rise in CRP was less pronounced at 5 hours for subjects consuming Rhodiolife. CRP returned to pre-test levels only in the RhodioLife group.
2. Similarly, elevated CK levels remained high 5 days post-test for both placebo and control groups but was significantly lower in the group supplemented with RhodioLife.
CLINICAL HIGHLIGHTS
Clinical Study: Rhodiolife increases physical working capacity
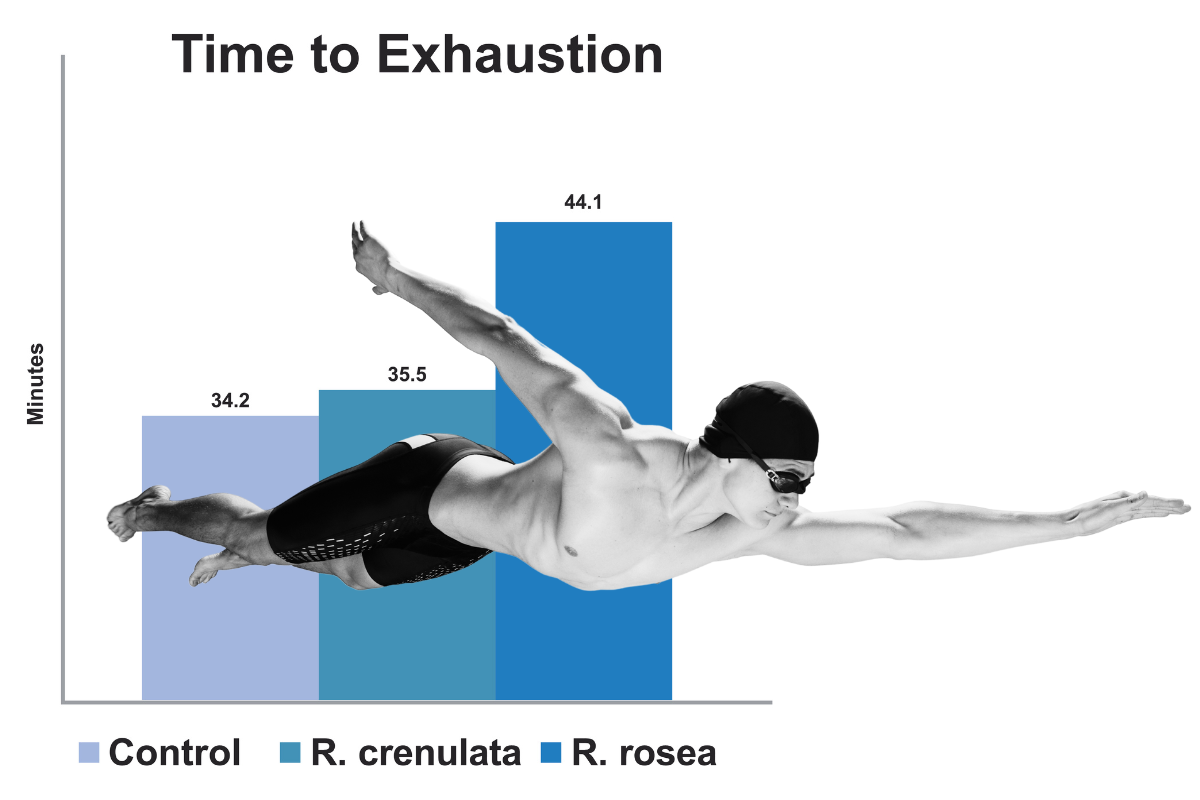
In this animal study, the effects of two different Rhodiola extracts were studied versus placebo on physical performance and muscle health during exhaustive swimming twice daily for 6 days. Subjects with Rhodiolife were able to swim almost 25% longer than control or R. crenulata supplemented rats. The effects of R. rosea extract are due to the presence of rosavins and/or their complex with other compounds. Abidov M, Crendal F, Grachev S, Seifulla R, Ziegenfuss T. Effect of Extracts from Rhodiola Rosea and Rhodiola Crenulata (Crassulaceae) Roots on ATP Content in Mitochondria of Skeletal Muscles. Bull Expo Biol Med. 2003;136(6):585–7.

Clinical Study: Rhodiolife supports muscle during exercise
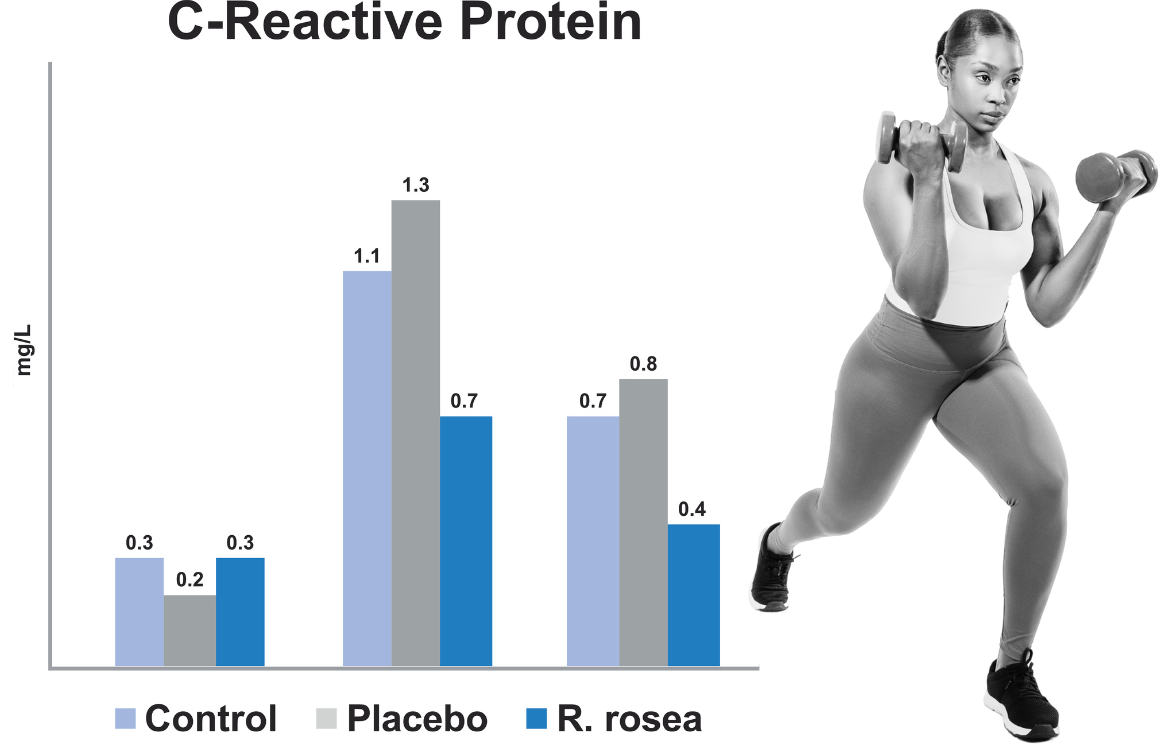
Untrained human volunteers (3 groups) were supplemented 30 days prior and 6 days after exhausting physical exercise using bicycle ergometer with: placebo (340 mg twice/day), Rhodiola rosea (RhodioLife, 340 mg twice/day) and control (no treatment) A less pronounced rise in the inflammatory marker C-reactive protein was observed in the RhodioLife supplemented subjects. Creatine Kinase, a marker of muscle breakdown or damage was also blunted in the RhodioLife supplemented subjects. Abidov, M & Grachev, S & Seifulla, R & Ziegenfuss, Tim. (2004) Bulletin of Experimental Biology and Medicine, 138. 63-4. 10.1023/B:BEBM.0000046940.45382.53.

Clinical Study: Rhodiolife may reduce occasional anxiety
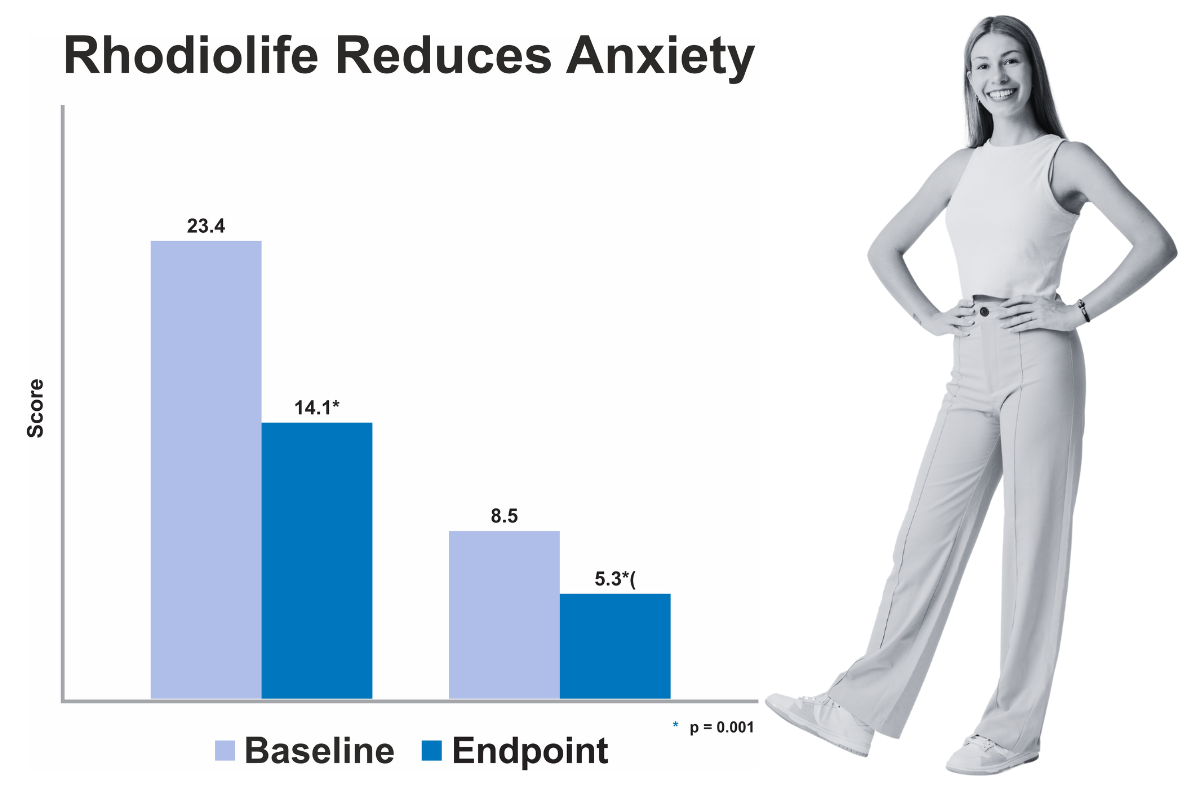
In this small, pilot study, 10 participants with DSM-IV diagnosis of generalized anxiety disorder were supplemented with Rhodiolife for 10 weeks. Several assessments to clinically evaluate symptoms at baseline and following supplementation were used. Daily Rhodiolife consumption decreased ratings on scales of anxiety (HAM-A) and depression (HAM-D). (p=0.001). Self-rated scores on FDADS also significantly decreased (p=0.043). Bystritsky A, Kerwin L, Feusner JD. J Altern Complement Med 2008;14(2):175–80.

MARKET OPPORTUNITIES
ACTIVE NUTRITION
Rhodiolife supports sustained energy and promotes faster recovery.
IMMUNE HEALTH
Rhodiolife supports healthy biological responses necessary for healthy immune, cardiopulmonary and endocrine systems.
COGNITIVE AND MOOD SUPPORT
Studies have shown that Rhodiola rosea can impact anxiety, stress, cognition and other mood symptoms.
E-SPORTS
Gamers are looking for enhanced cognitive support as well as the ability to play for many hours in a row. Rhodiolife can help!
INNOVATIVE BEVERAGES
Rhodiolife is available in a water-soluble form. Studies with Rhodiolife and beverage formulations show that it meets industry standards for performance in processing and that color, flavor, and the bioactives content are shelf-stable.
SPORTS GRADE
In July 2019, we announced the availability of third-party BSCG Certified Drug Free® Rhodiolife. This rigorous testing program established Rhodiolife as the first sports certified Rhodiola on the market.
APPLICATIONS

CAPSULES
EFFERVESCENTS

POWDERS

STICK PACKS

TABLETS

BEVERAGES

CHEWS

GUMMIES
ORIGIN STORY & INSIGHTS
An ingredient sourced from the top of the world
Rhodiola rosea is a hardy plant well suited to the cold climate and high altitudes where it grows and is now farmed. The plants used to make Rhodiolife grow in the Altai Mountains of Central Asia. Rhodiola rosea has a tradition of use that dates back centuries.

A true adaptogen
Rhodiola is a true adaptogen. Originally coined by Dr. Nikolai Lazarev in 1969, the term “adaptogen” refers to a substance that increases non-specific resistance to stimuli potentially harmful to the organism and stress.

A foremost rhodiola expert
Plant biochemist Dr. Zakir Ramazanov, who was a founding partner of Nektium Pharma, was one of the first to bring Rhodiola rosea to the attention of the English-speaking world and promoted it through his extensive research on its health benefits, starting as early as 1979.

High quality, effective, most trusted
Launched 25 years ago, the Rhodiolife brand has earned a reputation as a high-quality and effective adaptogenic botanical extract. The ingredient is the subject of groundbreaking ID assessment and sustainability programs.

The industry’s most rigorous identity and sustainability programs
When it comes to Rhodiola rosea, identity and sustainability matter to your customers. We deliver on both.

SUSTAINABILITY
 The Move to Cultivation and Compliance
The Move to Cultivation and Compliance
Rhodiolife is the subject of one of the industry’s first commercial scale cultivation programs for Rhodiola rosea roots as source materials for its extracts. The result of more than a decade of effort, the project has achieved a physical and phytochemical profile identical to previously supplied wildcrafted material. The new cultivated material is grown not by seeds but by vegetative propagation with root rhizomes – a technique that is faster, more dependable and, crucially, ensures that the molecular composition of the cultivated Rhodiola rosea is identical to the wild-grown plant. Nektium has put into place a Quality Assurance system that monitors Good Agricultural and Collection Practices (GACPs) governing rules of production, harvesting, storage, and record keeping. These are accompanied by training programs and traceability and recall plans. Manually harvested, the Rhodiola roots are transported to Nektium’s facilities in the Canary Islands for further processing. Rhodiolife has also been certified non-GMO by the Non-GMO Verification Project.
In 2023, Rhodiolife became the first CITES compliant Rhodiola marketed in North America. In November 2022 CITES – the Convention on International Trade in Endangered Species of Flora and Fauna – approved a proposal to add Rhodiola spp. to Appendix II. This is a list of species that are subject to controls in international trade. Today, PLT has CITES-compliant Rhodiolife available for our customers. The expertise we have developed in this area will ensure we have adequate supplies of this material, and our customers can rest easy that the documentation is handled,
Rhodiolife is a standardized extract of Rhodiola rosea – grown in the mountainous territory near the Arctic Circle. It is considered a true adaptogen. Only Rhodiolife’s unique “fingerprint” composition consistently provides the spectrum of compounds found in the root of the plant that are responsible for its biological activity – including rosavin, rosarin, rosin and salidroside. With Rhodiolife, not only is the percentage of the primary actives the same as the root, but so is the ratio of actives. This is not the case with many other Rhodiola rosea ingredients on the market.
IDENTITY & QUALITY YOU CAN TRUST
Reaching New Heights in Rhodiola rosea Quality Assurance
The Nektium ID assessment program
Nektium’s Quality Assurance team conducts multiple identity tests on every batch of Rhodiolife, including macroscopic and sensorial analysis, the development of chromatographic profiles and independent DNA barcode analysis to ensure the authenticity of the raw material. A gentle extraction process is used to unlock the active ingredients while preserving the natural phytochemical profile of the root. The material is then standardized to provide precise levels of bioactive compounds. The resulting HPLC ‘fingerprint’ of the Rhodiolife extract is consistent from batch to batch and matches with that of the native root.
This approach offers greater peace of mind around authenticity, which is especially important when adulteration is suspected. Recently, the American Botanical Council’s. Nektium’s Quality Assurance team conducts multiple identity tests on every batch of Rhodiolife, including macroscopic and sensorial analysis, the development of chromatographic profiles and independent DNA barcode analysis to ensure the authenticity of the raw material. A gentle extraction process is used to unlock the active ingredients while preserving the natural phytochemical profile of the root. The material is then standardized to provide precise levels of bioactive compounds. The resulting HPLC ‘fingerprint’ of the Rhodiolife extract is consistent from batch to batch and matches with that of the native root.

Exclusively manufactured by Nektium, Spain.


“The global demand for Rhodiola rosea has rapidly increased, which has led to shortages of authentic material. Most harvesting comes from alpine regions and forests, where supplies can be depleted quickly without replanting and sustainability efforts. I applaud PLT and Nektium for taking the initiative to develop a sustainable supply. Rhodiolife may well be the most sustainable Rhodiola rosea on the market today.”
Trish Flaster CEO & Founder of Botanical Liaisons
RESOURCES
The Feeling Active Mood and Cognitive Support Report reviews a study of 546 people in five countries on a broad range of topics ranging from their thoughts on mental energy, stress, sleep and focus and how they impact the sports and active experience.
Explore the latest in cognitive support for well-being with PLT's ingredient platform. In this webinar, you'll discover the science behind cognitive ingredients and how to incorporate them into formulations.



PLT Health Solutions, Inc. announced that it has received non-GMO verification from the Non-GMO Project company for its Rhodiolife® Rhodiola rosea ingredient. The verification covers raw materials, manufacturing processes and logistics.

PLT Health Solutions, Inc announced that it has begun offering CITES-compliant Rhodiolife® Rhodiola rosea in North America. This new development will assure its Rhodiola customers a secure supply of a premium, highly sustainable product that meets all regulatory requirements.




Give your customers
peace of mind
Rhodiolife is the highest quality, most traceable, sustainable Rhodiola rosea on the market today. Get in touch to give them the best.
- Expertise
- Ingredient Solutions
- All
- Animal Health & Wellness
- Beauty from Within
- Joint & Bone
- Cardiovascular
- Cognitive Performance
- Energy
- Functional Foods & Beverages
- Healthy Aging & Longevity
- Hydration+
- Immune & Respiratory Health
- Men’s Health
- Muscle Health
- Pain & Mobility
- Plant-Based Nutrition
- Sexual Health
- Sleep
- Sports & Active Nutrition
- Stress & Mood
- Weight Management
- Women’s Health
- Applications
- Resources
- PLT People & Planet
- About
These products are not intended to diagnose, treat, cure or prevent disease. This website is for informational purposes only.














