-
Ingredient SolutionsQuickly narrow your search. Browse products in our sortable catalog. View Catalog
-
ApplicationsQuickly narrow your search. Browse products in our sortable catalog. View Catalog
-
ResourcesRecently Posted
-
PLT People & Planet
-
About
Our international network, passionate team of experts and extensive industry knowledge is what sets us apart.
 Seth FlowermanCEO
Seth FlowermanCEO
Dynagenix® Muscle + Joint Formula
OVERVIEW
Beverage-friendly Dynagenix® Muscle+Joint Formula is clinically demonstrated to provide joint and muscle health benefits in as little as 5 days from start.

5 Days
Dynagenix offers fast-acting joint health support
47% Improvement
Dynagenix offers more joint comfort at 30 days

Up to 47% Less Muscle Soreness
Dynagenix reported statistically significantly less soreness than placebo at every recovery point

46% Improvement
Dynagenix delivers for overall joint health at 30 days
FEATURES & BENEFITS
Potent low dose |
|
Five-day efficacy |
|
Reduced post-exercise muscle & joint soreness* |
|
Supports faster, better recovery |
|
47% improvement in joint comfort at 30 days* |
|
47% reduction in joint stiffness at 30 days* |
|
45% improvement in joint function at 30 days* |

CERTIFICATIONS
VEGAN
GLUTEN-FREE
WATER SOLUBLE
NON-GMO
HALAL
KOSHER
A high bioavailability, water-soluble Boswellia-based ingredient
The starting point for Dynagenix – Boswellia serrata – is the Indian Frankincense tree, that produces AKBA-rich oleo-gum resin. Dynagenix provides a high concentration of AKBA, standardized to 78%.
Boswellia serrata
Sourced from the forests of India, Boswellia has been used in Ayurvedic medicine for thousands of years.

A Sacred Tree
Boswellia serrata are considered sacred by Indian families and given as gifts at weddings.
Rhodiola rosea
Rhodiola rosea is a plant native to remote Arctic climates in Asia, Europe and North America.

Exciting Phytochemistry
The root of the plant contains around 140 chemical compounds including phenols, rosavin, rosarin, salidroside and more.
Detail 1
Kale chips cold-pressed put a bird on it mumblecore kogi brooklyn farm-to-table blue bottle yuccie authentic kombucha migas. Literally tilde tacos paleo.

Detail 2
Kale chips cold-pressed put a bird on it mumblecore kogi brooklyn farm-to-table blue bottle yuccie authentic kombucha migas. Literally tilde tacos paleo.
RESEARCH
Clinical Science Demonstrates Wide Ranging Musculoskeletal Benefits
Dynagenix has been the subject of two clinical studies that examined various aspects of musculoskeletal support on diverse populations.
Rapid Improvement in Joint Function and Comfort for Everyone
In a 2025 clinical study, 72 men and women (age 40-70) with Grade II osteoarthritis, received either 40 mg/day of Dynagenix or a matching placebo for 30 days. Key outcome measures tested were WOMAC pain, stiffness, and function; a 30-second Chair Stand Test; a 6-minute Walk Test; a Stair Climb Test; and knee flexion and range of motion. Biomarkers of inflammation were also evaluated. Subjects were assessed at baseline, 5, 15, and 30 days.
This study showed that Dynagenix delivered statistically significant improvements in joint comfort, stiffness, and function as early as 5 days.

Dynagenix subjects showed statistically significant improvements in joint comfort, stiffness, and function as early as 5 days after supplementation, with a 45.6% Improvement in Total WOMAC score at 30 days.

At Day 30, Dynagenix subjects improved distance walked in 6 minutes by 47 meters, an improvement in walking speed of 8 meters per minute.

Dynagenix subjects significantly improved performance in a 30-Second Chair Stand test – a widely used diagnostic tool of physical function – at 2 weeks

Dynagenix subjects showed a substantial improvement in stair speed of fifty recreationally active men (age 25-40) were studied for their response to exercise-related muscle and joint soreness. The supplementation period was 10 days with a downhill running exercise on day 7. Recovery was assessed 24, 48, and 72 hours post-exercise, the period in which exercise-induced delayed-onset muscle and joint soreness occur.
Active/Sports Performance in a Younger Demographic
In a 2019 clinical trial, 50 recreationally active men (age 25-40) were studied for their response to eccentric exercise-related Delayed-Onset Muscle Soreness (DOMS) using 60mg of Dynagenix versus a placebo. The supplementation period was 10 days with eccentric exercise intervention on day 7, after which recovery was measured on d 8, 9 & 10.
The study demonstrated that daily supplementation of Dynagenix for 10 days results in a significant reduction in muscle soreness during squats (muscle recovery) in healthy male participants over placebo. In parallel, Dynagenix supplementation also improved the recovery of strength (1RM leg extension) and knee range of motion when compared to placebo. Dynagenix supplementation also alleviated the participants' knee soreness (joint recovery).
%2050%25%20Resize.png?width=1200&length=1200&name=Muscle%20Soreness%20(during%20squat)%2050%25%20Resize.png)

%2050%25.png?width=1200&length=1200&name=Joint%20Soreness%20(during%20squat)%2050%25.png)

The Borg rating of perceived exertion scale (RPE), a commonly used method scale to assess felt effort, clearly showed statistically significant benefits due to improvements in those taking Dynagenix compared to placebo at all time points.
CLINICAL HIGHLIGHTS
Clinical Study: Rapid Improvement in Joint Function
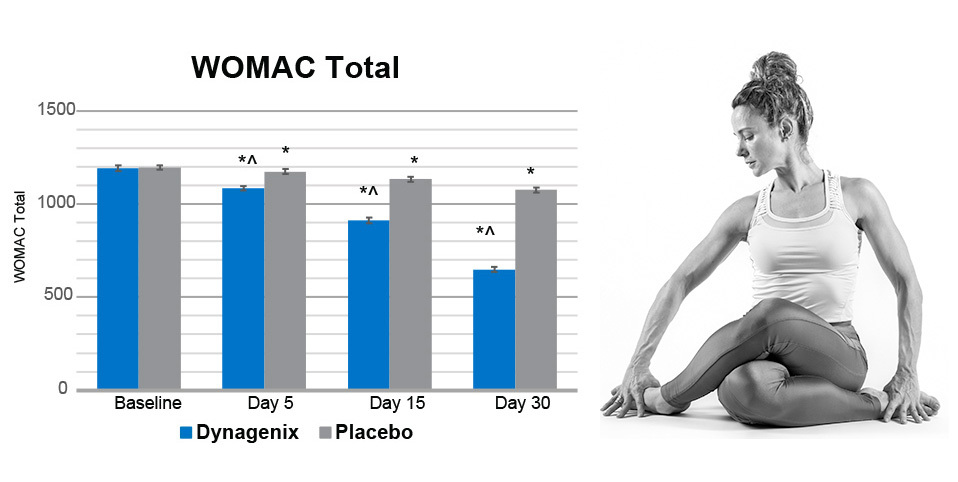
72 men and women (age 40-70) with Grade II osteoarthritis, received either 40 mg/day of Dynagenix or placebo for 30 days. The study demonstrated improved joint comfort, stiffness and function starting at 5 days.

Clinical Study: Reduced Joint & Muscle Soreness, Faster Recovery
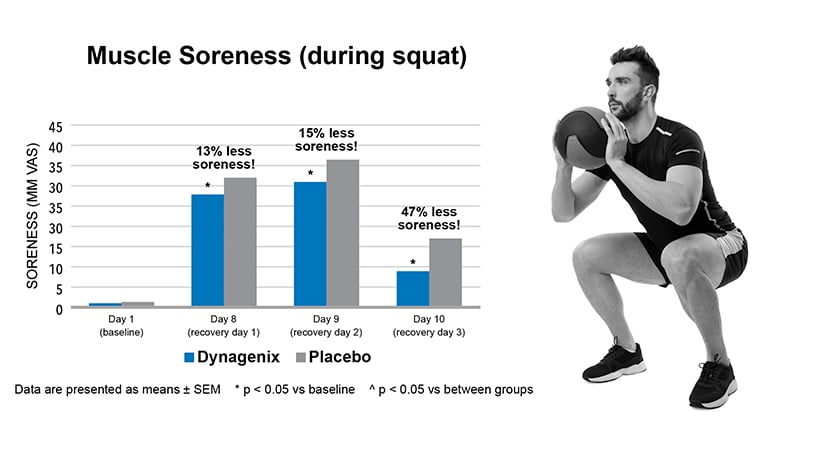
50 recreationally active men (age 25-40) were studied for their response to eccentric exercise-related Delayed-Onset Muscle Soreness (DOMS) using 60mg of Dynagenix versus a placebo. The study demonstrated reduced joint and muscle soreness and enhanced recovery.

MARKET OPPORTUNITIES
Active Nutrition
Dynagenix offers a comprehensive solution to post-exercise recovery and promotes the ability to perform at a higher level sooner.
Bone, Joint & Muscle Health
Beyond reducing post-activity muscle and joint soreness, Dynagenix can make everyday tasks easier – like even walking stairs.
APPLICATIONS

BEVERAGES

CAPSULES

CHEWS
EFFERVESCENTS

GUMMIES

POWDERS

RTDS/SHOTS

SOFT GELS

STICK PACKS

TABLETS
ORIGIN STORY
Sustainability Combined with Technology Development
Dynagenix is based on a proprietary Boswellia serrata gum resin extract, which you and your customers can be proud of. PLT Boswellia serrata materials are sourced in India from the states of Andhra Pradesh and Madhya Pradesh.

Long Established Partner
Over the last 25 years, PLT has worked with our innovation partner – Laila Nutraceuticals – to create an environment that can assure not only the highest quality ingredient with proven efficacy, but an ingredient that the people who sell it and buy it can be proud of.

Responding to the Industry
The Dynagenix product form was developed in response to industry requests for a more organoleptically favorable Boswellia ingredient. Most Boswellia extracts are completely insoluble. They also exhibit a strong odor and bitter taste.

A Variety of Delivery Systems
The development of this new form was a major technological achievement. Its water solubility and neutral taste properties open the ingredient up to inclusion into a wide range of liquid and solid delivery systems.

High Bioavailability
One of the surprises of this development process was that the final product showed improved bioavailability compared to the regular Boswellia extracts containing equivalent strength of AKBA.

QUALITY

From Harvest to Finished Ingredient
The starting point for Dynagenix – Boswellia serrata – is a plant that produces Indian frankincense. It is also known as Indian olibanum, Salai guggul, and Sallaki in Sanskrit. The plant is native to much of India and the Punjab region that extends into Pakistan. In partnership with Laila, PLT is committed to the safety and fair treatment of the people who harvest Dynagenix. We want to support the long-term health and well-being of the tribal culture and the natural environment upon which it relies.
Every batch of the Dynagenix harvest is assigned a specific batch number, and each batch of finished product is traced to a specific region and set of trees. Dynagenix is tested and must comply with predefined specifications for different parameters at different stages of product manufacturing – from raw materials to finished product. Quality is monitored by a multi-disciplinary organization with dedicated departments for taxonomy (botanical Identification), raw material analysis, chemical analysis, microbiology, and more.
SUSTAINABILITY
Understanding the Difference Makes the Difference
During the fourth quarter of 2021, PLT Health Solutions announced the commencement of a third-party sustainability audit program on Boswellia serrata trees, which serve as the raw material source for some of our most important ingredients. The audit of this India resource was conducted by renowned botanical research consultancy Botanical Liaisons, LLC, and was undertaken to help the nutraceutical industry and non-governmental agencies around the world understand what we and our ingredient innovation partner Laila Nutraceuticals have known for many years: proper stewardship of natural resources and social responsibility toward the communities that support our work are both the right thing to do and good business.
The audit program was a step in PLT’s campaign to document the sustainability of the tree resource. PLT’s Chairman, Paul Flowerman, made field trips to the key production areas in 2018 and 2019 to observe and document the variety of tree populations, methods of natural and forced propagation and the status of the tribal groups involved in the care of the trees and resin production.


A third audit program was based on a broad range of environmental, cultural and economic parameters. In March 2022 Botanical Liaisons reported to PLT its preliminary conclusions, which included this statement: “It is clear from the information collected from the stakeholders that [sustainability] is supported. Boswellia serrata has several sustainability advantages that prevent over-harvesting of PLT-sourced Boswellia compared to other sources and species of Boswellia.” The report recommended a series of next steps to further evaluate and promote sustainability.
Subsequently, four important additional developments have added considerable strength to the Boswellia serrata sustainability story:
- Authoritative tree survey data from Madhya Pradesh quantified the robustness and sufficiency of the tree resource to provide present and future resin requirements.
- CITES (Convention on International Trade in Endangered Species of Wild Fauna and Flora) determined during its November 2022 meeting (COP 19 in Panama City) that no Boswellia species would get added to the CITES Appendix lists. The health of Boswellia serrata, the only species of commercial importance for PLT, was documented, including information about the replenishment of the resource.
- AHPA (American Herbal Products Association) convened an expert panel, which has drafted a brochure entitled, “Good Stewardship Harvesting of Boswellia (Boswellia Serrata).” This is a definitive presentation of current best practices.
- An important study modeling the impact of significant climate change by 2050 on Boswellia serrata resources predicted that almost all of Madhya Pradesh, the source of 90+% of commercial resin, will remain conducive for a healthy tree population.
These initiatives represent the type of work we do with our PLT360™ initiative. Introduced in 2015, our PLT360 initiative is a business-wide commitment by PLT Health Solutions to develop ingredients that our customers can be confident and proud to supply to their own customers – knowing that these ingredients are safe, of high quality, efficacious and harvested and manufactured sustainably. PLT360 examines every business decision and business process we undertake to be transparent with our operations, build trust with the health & well-being community and, together with them, support healthier, happier lives for the consumers we serve.
INSIGHTS

PLT Mobility Solutions
At PLT, we are working on the concept of Mobility Solutions - making these solutions available for people of all ages and walks of life. We’re thinking about athletes who want to perform better and recover faster. We’re thinking about people who have physically demanding jobs who don’t want to or can’t take a day off from work. We’re thinking about anyone who has an active lifestyle who wants to embrace it despite the rigors. And yes, we’re thinking about the global aging population.
PLT Health Solutions’ leading portfolio of mobility support ingredients – based on more than a decade of ongoing research – has been developed to help you deliver innovative products that can capture the attention and the trust of consumers. All of our ingredients are backed by multiple clinical studies – and offer rapid improvements from a low dose - in just about any delivery system you can imagine.

“Dynagenix is exciting because it works at an exceptionally low dose and is water-soluble. This opens up a new world of innovation options for product formulators.”
Seth Flowerman, CEO, PLT Health Solutions
RESOURCES
PLT Health Solutions Unveils Second Clinical Study on Beverage-Friendly Dynagenix® Muscle+Joint Formula Demonstrating Enhanced Mobility & Rapid Joint Comfort within Five Days
Discover new mobility solutions for all demographics with clinically supported botanical ingredients. Watch the webinar for insights and a groundbreaking clinical study.
A breakthrough ingredient for active nutrition and more
The combination of benefits offered by Dynagenix and its ability to be delivered in multiple product forms can energize your product development efforts.
- Expertise
- Ingredient Solutions
- All
- Animal Health & Wellness
- Beauty from Within
- Joint & Bone
- Cardiovascular
- Cognitive Performance
- Energy
- Functional Foods & Beverages
- Healthy Aging & Longevity
- Hydration+
- Immune & Respiratory Health
- Men’s Health
- Muscle Health
- Pain & Mobility
- Plant-Based Nutrition
- Sexual Health
- Sleep
- Sports & Active Nutrition
- Stress & Mood
- Weight Management
- Women’s Health
- Applications
- Resources
- PLT People & Planet
- About
These products are not intended to diagnose, treat, cure or prevent disease. This website is for informational purposes only.














